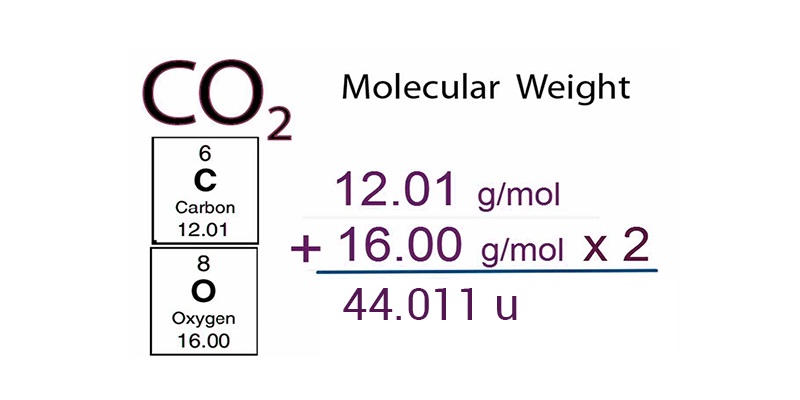
Molecular Mass:
The Molecular mass of a substance is the sum of the atmoic masses of all the atoms in a molecule of the substance.
Formula Unit Mass:
The formula unit mass of a substance is a sum of the atmoic of all atmos in a formula unit of a compound. Formula unit mass is calculated in the same manner as we calculate the molecular mass.The only difference is thatwe use the word formula unit for those substances whose constituent particlesare ions.
Mole Concept:
A mole is defined as the amount of a substance that contains exactly the Avogadro number of 'elementary entities' of the given substance. Moles=Mass of Sample / Molar Mass. You can calculate the total number of atoms/molecules in a sample by multiplying the number of moles by the Avogadro constant. The formula is: Atoms or Molecules=Number of Moles / 6.022*10 23 .