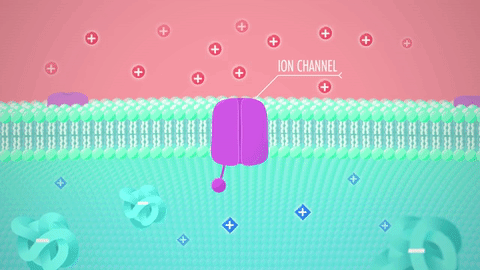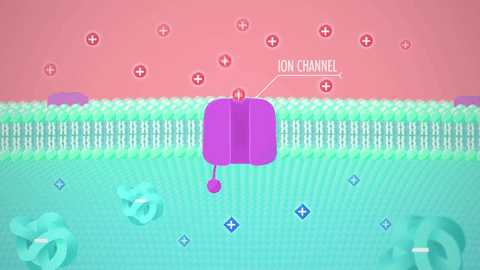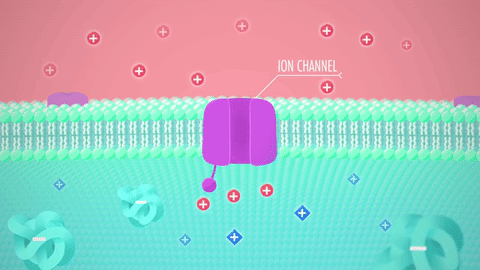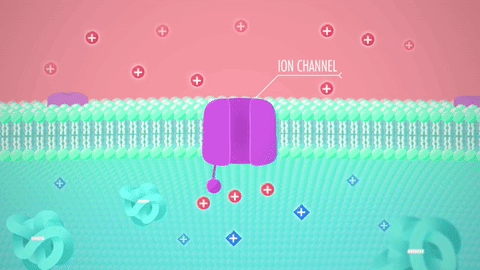
What are these structures:
An ion is a charged atom or molecule. It is charged because the number of electrons do not equal the number of protons in the atom or molecule.
A cation is a positively charged ion with fewer electrons than protons while an anion is a negatively charged ion with more electrons than protons.
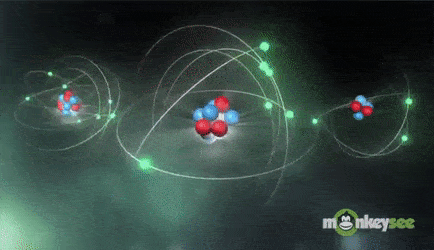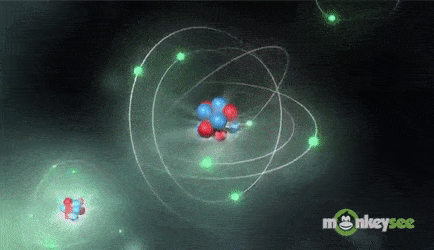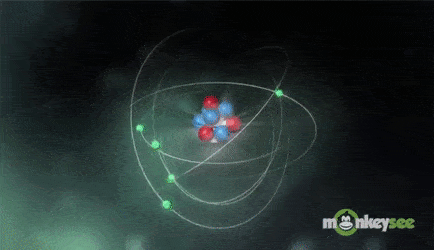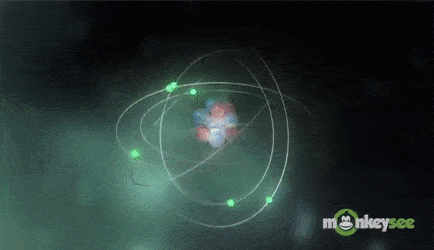
Ions are formed when the number of protons in an atom does not equal the number of electrons. An ion therefore is an atom that has gained or lost one or more electrons and therefore has a negative or positive charge. Ionization is the process of exchanging electrons among atoms or molecules.
There are two types of ions: cations and anions. A cation has a net positive electrical charge, which means it has more protons than electrons. An anion has a net negative electrical charge, which means it has more electrons than protons.
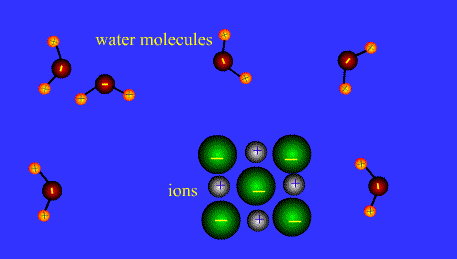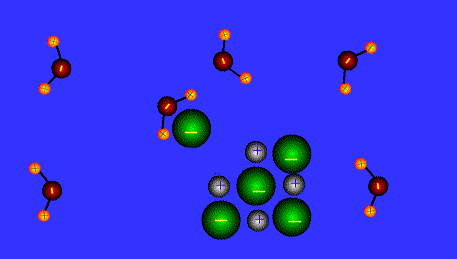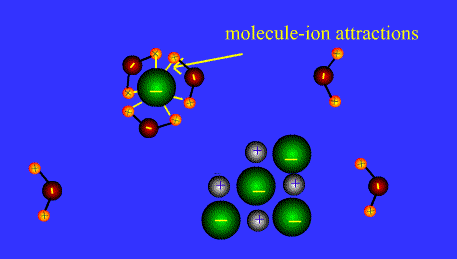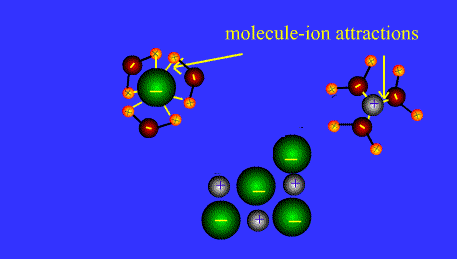
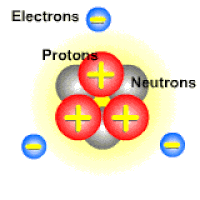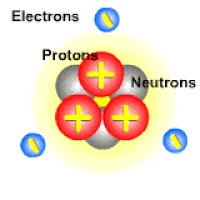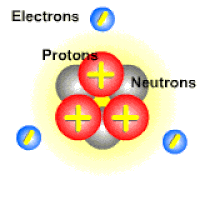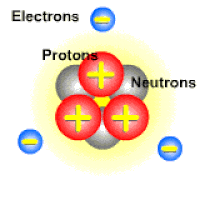
Ions form when doing so minimizes the total potential energy of the chemical species involved in the chemical reaction. Often, this is achieved by allowing atoms of different elements to achieve a full shell of electrons. Consider the salt lithium fluoride: Lithium atoms have 3 electrons, meaning they have electrons in two shells: 2 in the first shell and 1 in the second. By losing an electron to become a cation, lithium gets a stable electron arrangement, identical to that of the noble gas helium.