Laws of Chemical Combination:
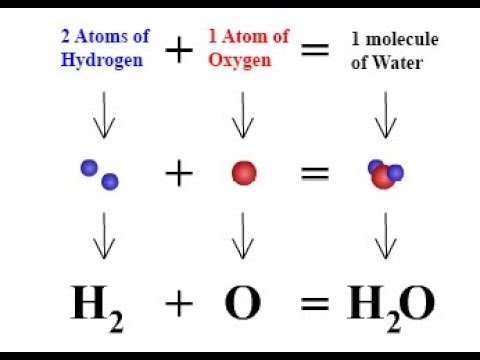
It states that"A chemical compound is always found to be made up of the same elements combined together in the same fixed proportion by mass".
For example,a sample of pure water from various sources or any country is always made up of only hydrogen and oxygen.
The laws of chemical combination describe the basic principles obeyed by interacting atoms and molecules,interactions that happen in many different ways.
Law of Conversation of Mass:
In simple terms, this law states that matter can neither be created nor destroyed.
Law of Definite Proportions.
Law of multiple proportions.
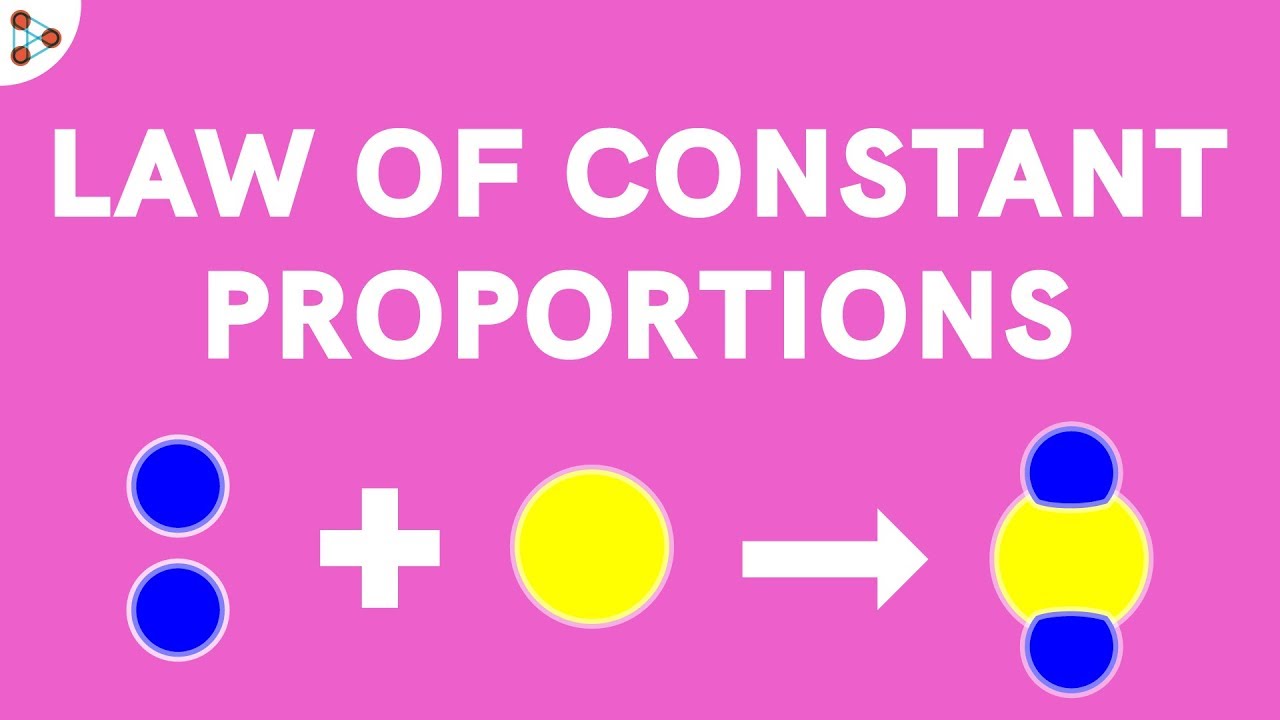
Laws of Constant Proportion:
The law of constant proportions states that chemical compounds are made up of elements that are present in a fixed ratio by mass.This implifies that any pure sample of a compound, no matter the source,will always consist of the same elements that are present in the same ratio by mass.