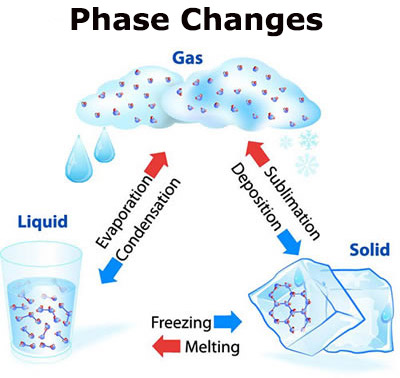
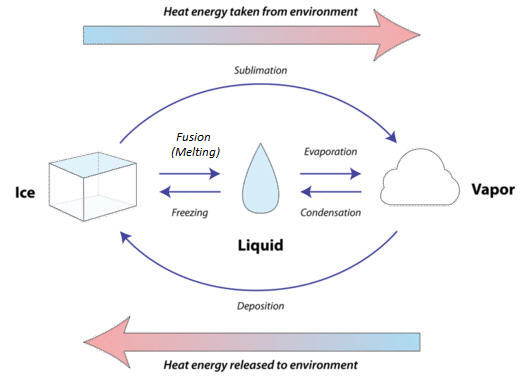
Matter changes from one state to another by heating, cooling or by other methods. On heating the intermolecular space between the molecules increases and the intermolecular force decreases in solids and liquids and then changes the state to liquid in case of solid and gas in case of liquid During the cooling process the intermolecular space decreases whereas intermolecular force decreases between the molecules The matter changes from one state to another in the following ways:-
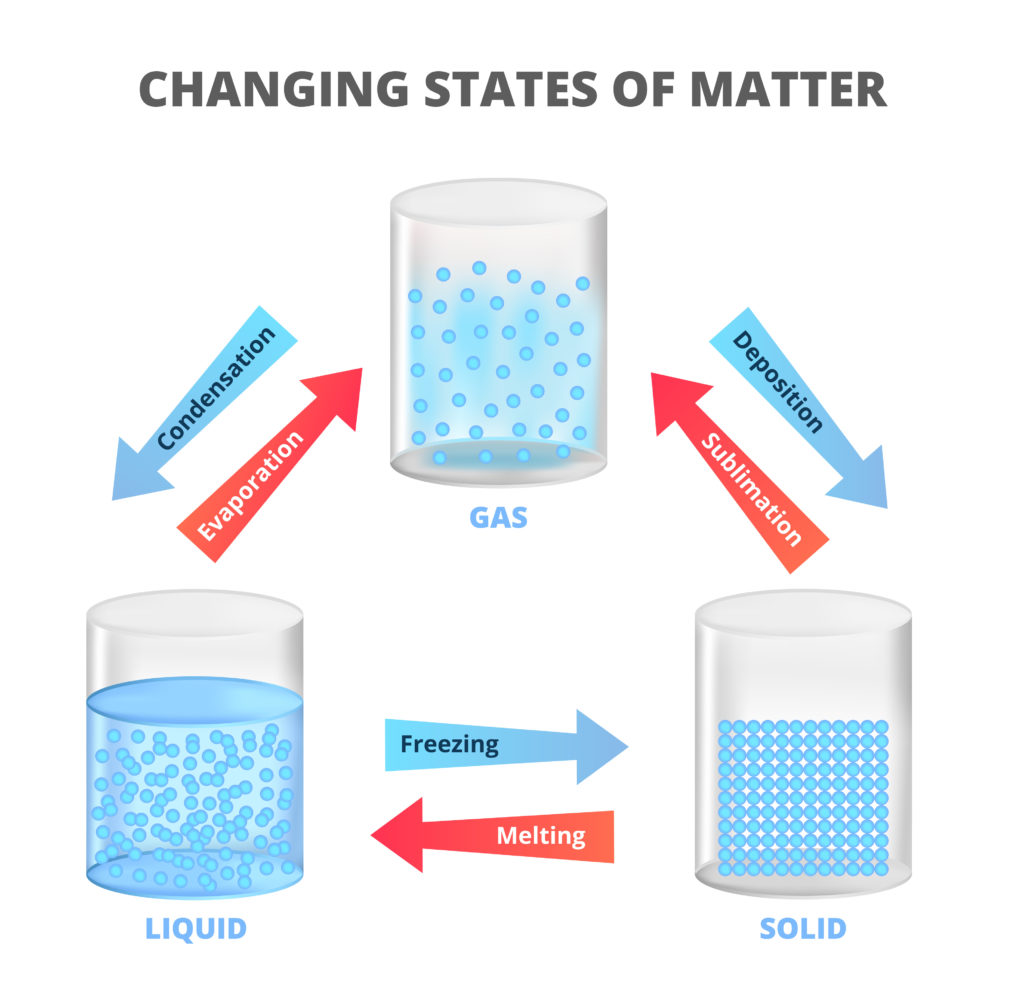
Changing states of matter occur when matter loses or absorbs energy. When a substance absorbs energy; the atoms and molecules move more rapidly and this increased kinetic energy pushes particles far enough that they change form.