Overview
Welcome to the fascinating world of Heat! In this section, we will explore the basic concepts of heat and its various forms of transfer. Understanding heat is crucial as it plays a significant role in our daily lives and has widespread applications in different fields of science and technology.
Topics
-
Heat
Heat is one of the essential forms of energy for the survival of life on earth. Transfer of heat takes place from one body to another due to differences in temperature as per thermodynamics. We use heat energy for various activities like cooking, ironing, transportation, recreation, etc. This form of energy also plays a vital role in nature. The occurrence of the wind, rain, change of seasons, etc., depends on the gradient created due to uneven heating of different regions. Heat is defined as the flow of energy from a warm to a cooler object. The direction of flow of the heat energy takes from the substance of higher temperature to the substance of lower temperature. This is because the molecules are vibrating faster and transfer their energy to the molecules vibrating slower. The vibrational energy is also termed as its heat content. The heat content in the body makes it hot or cold. Greater the heat content, the hotter the body will be.
-
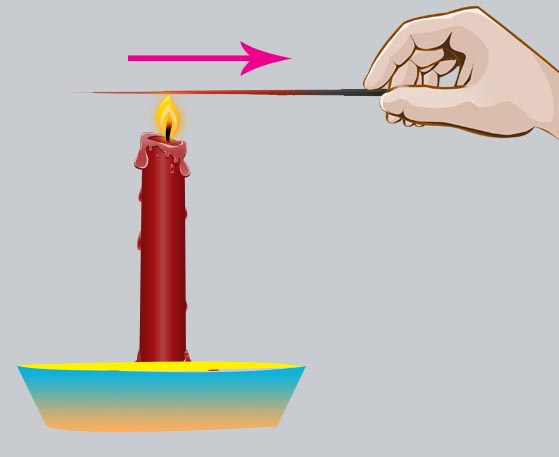
Conduction
Conduction, in general, is the process of transmission of energy from one particle of the medium to the other, but here, each particle of the medium stays at its own position. In Physics and Chemistry, the meaning of conduction is understood mainly as the transfer of heat energy or an electric charge through a material. Conduction can occur in solids, liquids, and gases. When conduction of heat occurs, the heat energy is usually transferred from one molecule to another molecule as they are in direct contact with each other. However, there is no change in the position of the molecules. They simply vibrate amongst each other. During the conduction of electricity, there is a movement of electrically charged particles in the medium. As such, the electric current is usually carried and moved by the ions or electrons.
-
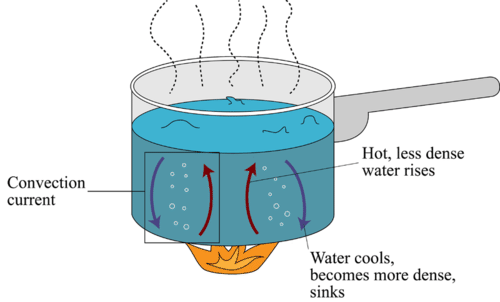
convection
Convection is the process of heat transfer by the bulk movement of molecules within fluids such as gases and liquids. The initial heat transfer between the object and the fluid takes place through conduction, but the bulk heat transfer happens due to the motion of the fluid. Convection is the process of heat transfer in fluids by the actual motion of matter. It happens in liquids and gases. It may be natural or forced. It involves a bulk transfer of portions of the fluid.
Types Of Convection
There are two types of convection, and they are: Natural convection Forced convection Natural convection: When convection takes place due to buoyant force as there is a difference in densities caused by the difference in temperatures it is known as natural convection. Examples of natural convection are oceanic winds. Forced convection: When external sources such as fans and pumps are used for creating induced convection, it is known as forced convection. Examples of forced convection are using water heaters or geysers for instant heating of water and using a fan on a hot summer day.
-
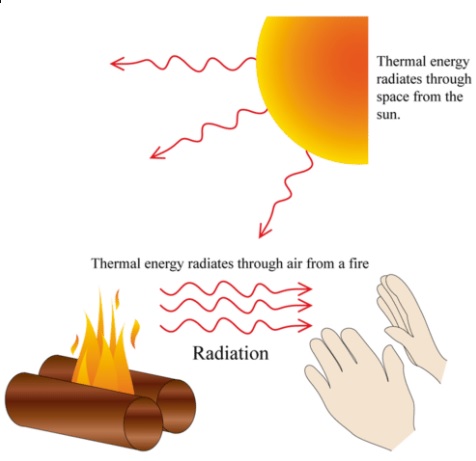
Radiation
Radiation can be described as energy or particles from a source that travels through space or other mediums. Light, heat, microwaves and wireless communications are all forms of radiation. This includes the following: Particle Radiation: such as alpha radiation (α), beta radiation (β), and neutron radiation. Gravitational Radiation: such as radiation that takes the form of gravitational waves, or ripples in the curvature of space-time. Acoustic Radiation: such as ultrasound, sound, and seismic waves. Electromagnetic radiation: such as radio waves, visible light, x-rays, and gamma radiation.
Types Of Radiation
Radiation is often categorized into two types depending on the energy of the radiated particles. Ionizing Radiation Ionizing radiation carries more than 10 eV, which is enough to ionize atoms and molecules and break chemical bonds.The ionizing radiation consists of alpha particles, beta particles, and gamma rays. Non-ionizing Radiation Ionization is not caused by these radiations. They usually emit heat, which can sometimes be so intense as to result in burns. Visible light and infrared radiation are examples of non-ionizing radiation that may be seen by humans.