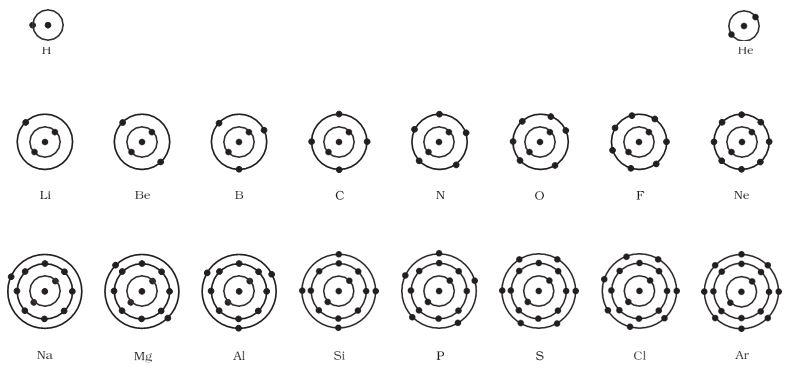
The distribution of electrons into different orbits of an atom was suggested by Bohr and Bury.Neils Bohr gave the planetary model of an atom. He was the first person to suggest the periodicity in the properties of the elements. “Bohr atomic model” forms the basis of the electronic structure of an atom. He was the person to describe the arrangement of electrons (electronic configuration) in different orbits/shells. He proposed that electrons are distributed in circular electronic shells (orbits). These electrons revolve in the orbits around the nucleus from a fixed distance. In this topic, we will learn more about the electronic configuration of different elements. Atomic structure of the first eighteen elements is shown in below figure

The distribution of electrons in an atom is called Electronic Configuration. Formula 2n2 helps in the determination of the maximum number of electrons present in an orbit, here n= orbit number. The formula helps in the determination of the arrangement of electrons and is known as “Bohr Bury Schemes". Electrons are negatively charged subatomic particles arranged like a cloud of negative charges outside the nucleus of an atom. The arrangement depends upon their potential energies in different orbits. The different energy levels are known as 1, 2, 3, 4….. and the corresponding shells are known as K, L, M, N and so on.

The shells begin from the centre and gradually move outwards. So K shell will always have minimum energy. Similarly, the L shell is a little away from the nucleus so it will have higher energy than the K shell. The outermost shell will have maximum energy. Now it is important to understand the distribution and arrangement of electrons in the atoms of any elements in the different energy levels. An atom of any element is most stable when it has minimum energy. An atom will first fill the lowest energy level so as to attain the state of minimum energy. Gradually, the electrons will fill the higher energy levels. Therefore, electrons will first fill the K shell, then the L shell, M shell, N shell, and so on.
