Those elements that have the same atomic number but a different mass number are referred to as isotopes. Isotopes occur due to the presence of a different number of neutrons in elements having the same atomic number as mass number is the sum of the number of neutrons and protons. Many but not all elements have isotopes. The isotopes of hydrogen are protium (has one proton and no neutrons), deuterium (has one proton and one neutron) and tritium (has one proton and two neutrons). The chemical properties of isotopes are the same owing to the fact that they have the same number of protons and hence the same number of electrons which determines the chemical properties of an element.
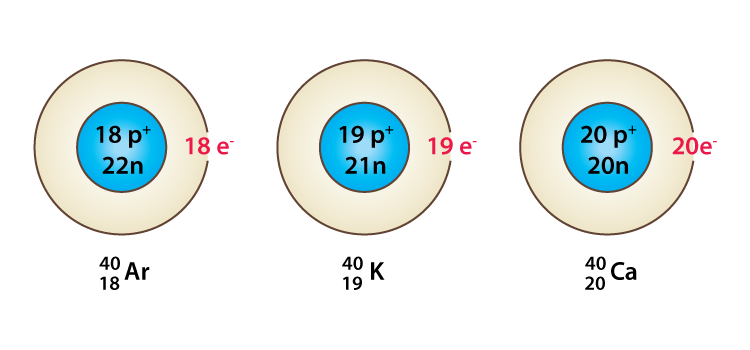
Isobars, on the other hand, are the atoms having the same mass number but a different atomic number. For example, the atomic number of Argon is 18 and mass number is 40, the atomic number of Potassium is 19 and the mass number is 40 similarly the atomic number of Calcium is 20 and mass number is 40. Argon, Potassium and Calcium are the same mass numbers but different atomic numbers so they are Isobars with each other. Similarly the atomic number of carbon and nitrogen is 6 and 7 respectively. Carbon-14 an isotope of carbon has a mass number of 14 which is same as that of nitrogen and hence carbon-14 and nitrogen are isobars.