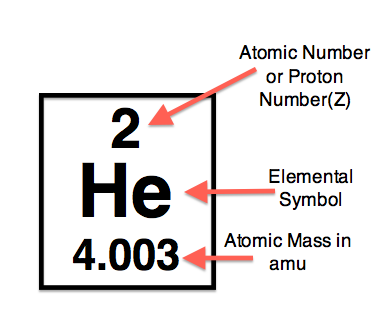
The total number of protons in the nucleus of an atom gives us the atomic number of that atom. It is represented with the letter ‘Z.’ All the atoms of a particular element have the same number of protons, and hence the same atomic number. Atoms of different elements have different atomic numbers.
In other words,the number of protons in the nucleus of an atom determines an element's atomic number. Each element has a unique number that identifies how many protons are in one atom of that element. For example, all hydrogen atoms, and only hydrogen atoms, contain one proton and have an atomic number of 1. All carbon atoms, and only carbon atoms, contain six protons and have an atomic number of 6. Oxygen atoms contain 8 protons and have an atomic number of 8. The atomic number of an element never changes, meaning that the number of protons in the nucleus of every atom in an element is always the same.

Mass number is the number of nucleons (the sum of protons and neutrons) present in an atom. Mass number is also called as atomic number or nucleon number.Rutherford showed that the vast majority of the mass of an atom is concentrated in its nucleus, which is composed of protons and neutrons. The mass number is defined as the total number of protons and neutrons in an atom. It can be calculated by adding the number of neutrons and the number of protons (atomic number) together.
