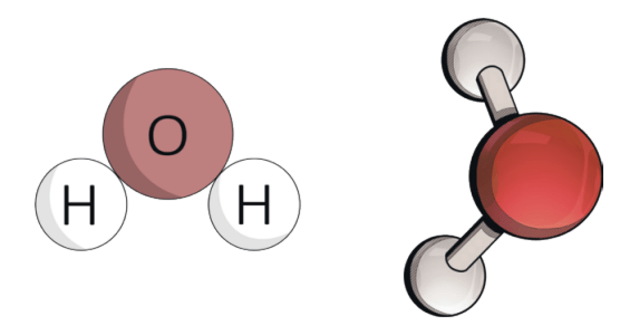Compounds
When two or more elements chemically combine in a fixed ratio by mass, the obtained product is known as a compound. Compounds can be defined as substances consisting of 2 or more different types of elements in a fixed ratio of their atoms. When the elements combine, some individual property of the elements is lost and the newly formed compound has new properties.Compounds are represented by their chemical formula. A chemical formula is a symbolic representation of the proportions of atoms that constitute a particular chemical compound.

Types of Compounds
- Organic compounds always contain carbon. If any carbon atoms in the compound bind to hydrogen, it is organic. Examples of organic compounds are:
Octane (C₈H₁₈)
Ethanol (C₂H₆O)
Glucose (C₆H₆O₆)

- Inorganic compounds do not have carbon, with certain exceptions. If a compound contains carbon, but the carbon atoms do NOT bond to any hydrogen atoms, it is inorganic. For example, compounds that have carbonate (CO₃ −2) or cyanate (OCN−) ions are inorganic. Some inorganic compounds include:
Calcium carbonate (CaCO₃)
Carbon dioxide (CO₂)
Water (H₂O)

- Binary compounds can have more than two atoms, but precisely two elements make them up. There are many binary compounds, and they can be ionic or covalent, inorganic or organic.