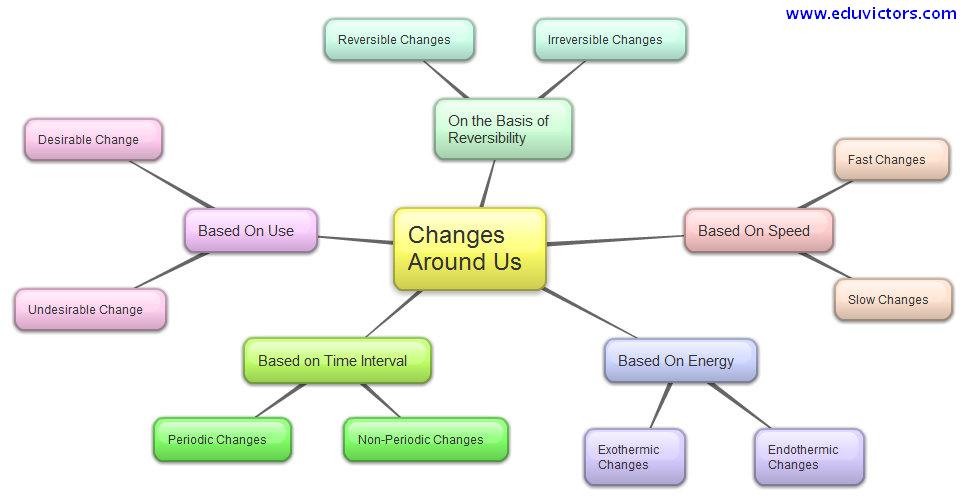
Changes Around US...
To study the ways a change is brought about.
It may be classified under the following:
Slow Changes:
These are the kinds of changes that will happen over a period of time slowly. For instance, a boy becoming a man is an example of this as it happens over the years.
Fast Changes:
As the name suggests, these changes occur sooner. For instance, a batter turning into pudding. It will take hardly a few minutes for a liquid batter to turn into a pudding.
Physical Change:
Those changes which may be reversed are Physical changes. For instance, changing water to ice and vice versa will be categorized under this.
Chemical Change:
A change that becomes permanent in nature is an Chemical change. For instance, when we burn paper it turns to ash and cannot be turned back to paper.
Therefore, this is permanent.
Question For You
Q. What is a chemical change? Explain it with the help of an example?
Ans: Any change which is a result of mixing two chemical compounds to form a new chemical compound is known as a chemical change.
Example of this can be that when you add four molecules of Sulphate to one molecule of Magnesium, it creates Magnesium Sulphate, that is more commonly
known as the Epsom salt.
Q. To walk through a waterlogged area, you usually shorten the length of your dress by folding it. Can this change be reversed?
Ans:Yes, the process can be reversed.
Q. You accidentally dropped your favorite toy and broke it. This is a change you did not want. Can this change be reversed?
Ans:No, this change cannot be reversed
Q.Some changes are listed in the following table. For each change, write in the blank column, whether the change can be reversed or not ?
| Sl No |
Change |
Can be reversed Yes/No |
| 1 |
The sawing of a piece of wood |
No |
| 2 |
The melting of ice candy |
Yes |
| 3 |
Dissolving sugar in water |
Yes |
| 4 |
The cooking of food |
No |
| 5 |
The ripening of a mango |
No |
| 6 |
Souring of milk |
No |
Q. A drawing sheet changes when you draw a picture on it. Can you reverse this change?
Ans:This change can be reversed if a pencil is used to draw the picture. If a pen, paint, oil /water colours are used to draw the picture,
change cannot be reversed.
Q. Give examples to explain the difference between changes that can or cannot be reversed.
Ans:Changes that can be reversed
i) Opening and closing the door
ii) Filling glass with water
changes that cannot be reversed
i) Conversion of milk into curd
ii) Ripening of fruit
Q. A thick coating of a paste of Plaster of Paris (POP) is applied over the bandage on a fractured bone. It becomes hard on drying to keep the fractured
bone immobilized. Can the change in POP be reversed?
Ans:No, the change cannot be reversed
Q. A bag of cement lying in the open gets wet due to rain during the night. The next day the sun shines brightly. Do you think the changes,
which have occurred in the cement, could be reversed?
Ans: No, the change cannot be reversed
Q.Difference between physical and chemical changes?
Ans:
| Physical Change |
Chemical Change |
|
A change in matter which occurs without causing any change in the composition of the matter is known as physical change
|
While a chemical change is defined as the change in the chemical composition of matter
|
|
Usually, physical changes are reversible in nature
|
While chemical changes are often irreversible
|
|
No new products are formed when an object undergoes physical change
|
Chemical changes often lead to formation of new products
|
|
These changes have no impact on the molecular composition of the substance
|
Chemical changes have a direct impact on the chemical bonds and molecular composition of a substance
|
|
A few changes occur when cooling or heating is done
|
These changes involve absorption or release of energy
|
There are other ways to bring about changes in substances:
Mixing two substances together: A small amount of curd is added to warm milk which leads to conversion of that milk into curd. This is an irreversible change.
When we add a salt to water it becomes salty but this is a reversible change.
Expansion and Contraction: In order to make tools like an axe, the ring of its iron blade is heated which allows it to expand i.e.
become larger in size and then is allowed to cool down which makes it contract again i.e. become smaller in size leading to a tight fit of the handle.
 Curd is added to milk to allow it to set into curd.
Curd is added to milk to allow it to set into curd.




 Curd is added to milk to allow it to set into curd.
Curd is added to milk to allow it to set into curd. 
